G.Patton
Expert
- Joined
- Jul 5, 2021
- Messages
- 2,440
- Solutions
- 3
- Reaction score
- 2,461
- Points
- 113
- Deals
- 1
Introduction.
Laboratory glassware refers to a variety of equipment used in laboratory work, and traditionally made of glass. Glass can be blown, bent, cut, molded, and formed into many sizes and shapes, and is therefore common in chemistry, biology, and analytical laboratories. Glassware in the laboratory comes in a range of different shapes and sizes, and is used for a number of purposes. Don’t know your round-bottomed flask from your Florence flask, or your pipettes from your burettes? This topic has you covered. You can find information about most usable in drug manufacturing laboratory glassware below. Each unit of glassware has descriptions and instructions.
Laboratory beakers and glass rods.
Beakers - high, low, thin-walled cylindrical containers with or without a spout with a capacity of 5 ml to 5 liters from different materials. Glasses are used for pouring liquids, preparing solutions, as receivers in various installations. It is impossible to heat glasses made of ordinary glass on a flame, because of this they burst. Heating of heat-resistant glasses should be carried out only in a water bath or any other (sand, oil bath). Heat-resistant glass can withstand temperatures up to 650 degrees.
(B) A tall-form or Berzelius beaker.
(C) A flat beaker or crystallizer.
Laboratory glass rods are designed for mixing solutions in glass laboratory glassware. Convenient for other manipulations with chemicals.
Test tubes.
Test tubes are narrow cylindrical vessels with a rounded bottom. They differ in diameter, height and materials. They are used for analytical and microchemical work. In addition, there are also graduated and centrifuge conical tubes. Test tubes intended for general chemical work are usually made of glass, for its relative resistance to heat. Tubes made from expansion-resistant glasses, mostly borosilicate glass or fused quartz, can withstand high temperatures up to several hundred degrees Celsius.
Chemistry tubes are available in a multitude of lengths and widths, typically from 10 to 20 mm wide and 50 to 200 mm long. The top often features a flared lip to aid pouring out the contents. A chemistry test tube typically has a flat bottom, a round bottom, or a conical bottom. Some test tubes are made to accept a ground glass stopper or a screw cap. They are frequently provided with a small ground glass or white glaze area near the top for labelling with a pencil. Test tubes are widely used by chemists to handle chemicals, especially for qualitative experiments and assays. Their spherical bottom and vertical sides reduce mass loss when pouring, make them easier to wash out, and allow convenient monitoring of the contents. The long, narrow neck of the test tube slows down the spreading of gases to the environment.
Test tubes are convenient containers for heating small amounts of liquids or solids with a Bunsen burner or alcohol burner. The tube is usually held by its neck with a clamp or tongs. By tilting the tube, the bottom can be heated to hundreds of degrees in the flame, while the neck remains relatively cool, possibly allowing vapours to condense on its walls. A boiling tube is a large test tube intended specifically for boiling liquids. A test tube filled with water and upturned into a water-filled beaker is often used to capture gases, e.g. in electrolysis demonstrations. A test tube with a stopper is often used for temporary storage of chemical or biological samples.
Chemistry tubes are available in a multitude of lengths and widths, typically from 10 to 20 mm wide and 50 to 200 mm long. The top often features a flared lip to aid pouring out the contents. A chemistry test tube typically has a flat bottom, a round bottom, or a conical bottom. Some test tubes are made to accept a ground glass stopper or a screw cap. They are frequently provided with a small ground glass or white glaze area near the top for labelling with a pencil. Test tubes are widely used by chemists to handle chemicals, especially for qualitative experiments and assays. Their spherical bottom and vertical sides reduce mass loss when pouring, make them easier to wash out, and allow convenient monitoring of the contents. The long, narrow neck of the test tube slows down the spreading of gases to the environment.
Test tubes are convenient containers for heating small amounts of liquids or solids with a Bunsen burner or alcohol burner. The tube is usually held by its neck with a clamp or tongs. By tilting the tube, the bottom can be heated to hundreds of degrees in the flame, while the neck remains relatively cool, possibly allowing vapours to condense on its walls. A boiling tube is a large test tube intended specifically for boiling liquids. A test tube filled with water and upturned into a water-filled beaker is often used to capture gases, e.g. in electrolysis demonstrations. A test tube with a stopper is often used for temporary storage of chemical or biological samples.
Graduated cylinders.
Cylinders are vessels with graduations marked on the outer wall, intended for measuring certain volumes of liquids during laboratory work. It has a narrow cylindrical shape. The cylinders are produced in four versions: a cylinder with a spout; cylinder with glass stopper; cylinder with a plastic stopper; cylinder with spout and plastic base; cylinder with plastic stopper and plastic base. In addition to cylinders, for the same purpose, beakers are used - conical vessels, on the walls of which there are divisions.
Pipettes and dispensers.
A pipette (sometimes spelled pipet) is a laboratory tool commonly used in chemistry, biology and medicine to transport a measured volume of liquid, often as a media dispenser. Pipettes come in several designs for various purposes with differing levels of accuracy and precision, from single piece glass pipettes to more complex adjustable or electronic pipettes. Many pipette types work by creating a partial vacuum above the liquid-holding chamber and selectively releasing this vacuum to draw up and dispense liquid. Measurement accuracy varies greatly depending on the instrument.
Air displacement pipettes.
Piston-driven air displacement pipettes are a type of micropipette, which are tools to handle volumes of liquid in the microliter scale. They are more commonly used in biology and biochemistry, and less commonly in chemistry; the equipment is susceptible to damage from many organic solvents.
These pipettes operate by piston-driven air displacement. A vacuum is generated by the vertical travel of a metal or ceramic piston within an airtight sleeve. As the piston moves upward, driven by the depression of the plunger, a vacuum is created in the space left vacant by the piston. Air from the tip rises to fill the space left vacant, and the tip air is then replaced by the liquid, which is drawn up into the tip and thus available for transport and dispensing elsewhere. Sterile technique prevents liquid from coming into contact with the pipette itself. Instead, the liquid is drawn into and dispensed from a disposable pipette tip that is changed between transfers. Depressing the tip ejector button removes the tip, that is cast off without being handled by the operator and disposed of safely in an appropriate container. This also prevents contamination of or damage to the calibrated measurement mechanism by the substances being measured. The plunger is depressed to both draw up and dispense the liquid. Normal operation consists of depressing the plunger button to the first stop while the pipette is held in the air. The tip is then submerged in the liquid to be transported, and the plunger is released in a slow and even manner. This draws the liquid up into the tip. The instrument is then moved to the desired dispensing location. The plunger is again depressed to the first stop, and then to the second stop, or 'blowout', position. This action will fully evacuate the tip and dispense the liquid. In an adjustable pipette, the volume of liquid contained in the tip is variable; it can be changed via a dial or other mechanism, depending on the model. Some pipettes include a small window which displays the currently selected volume. The plastic pipette tips are designed for aqueous solutions, and are not recommended for use with organic solvents that may dissolve the plastics of the tips or even the pipettes.
Air displacement pipettes.
Piston-driven air displacement pipettes are a type of micropipette, which are tools to handle volumes of liquid in the microliter scale. They are more commonly used in biology and biochemistry, and less commonly in chemistry; the equipment is susceptible to damage from many organic solvents.
These pipettes operate by piston-driven air displacement. A vacuum is generated by the vertical travel of a metal or ceramic piston within an airtight sleeve. As the piston moves upward, driven by the depression of the plunger, a vacuum is created in the space left vacant by the piston. Air from the tip rises to fill the space left vacant, and the tip air is then replaced by the liquid, which is drawn up into the tip and thus available for transport and dispensing elsewhere. Sterile technique prevents liquid from coming into contact with the pipette itself. Instead, the liquid is drawn into and dispensed from a disposable pipette tip that is changed between transfers. Depressing the tip ejector button removes the tip, that is cast off without being handled by the operator and disposed of safely in an appropriate container. This also prevents contamination of or damage to the calibrated measurement mechanism by the substances being measured. The plunger is depressed to both draw up and dispense the liquid. Normal operation consists of depressing the plunger button to the first stop while the pipette is held in the air. The tip is then submerged in the liquid to be transported, and the plunger is released in a slow and even manner. This draws the liquid up into the tip. The instrument is then moved to the desired dispensing location. The plunger is again depressed to the first stop, and then to the second stop, or 'blowout', position. This action will fully evacuate the tip and dispense the liquid. In an adjustable pipette, the volume of liquid contained in the tip is variable; it can be changed via a dial or other mechanism, depending on the model. Some pipettes include a small window which displays the currently selected volume. The plastic pipette tips are designed for aqueous solutions, and are not recommended for use with organic solvents that may dissolve the plastics of the tips or even the pipettes.
A volumetric pipette, bulb pipette, or belly pipette allows extremely accurate measurement (to four significant figures) of the volume of a solution. It is calibrated to deliver accurately a fixed volume of liquid. These pipettes have a large bulb with a long narrow portion above with a single graduation mark as it is calibrated for a single volume (like a volumetric flask). Typical volumes are 1, 2, 5, 10, 20, 25, 50 and 100 mL. Volumetric pipettes are commonly used in analytical chemistry to make laboratory solutions from a base stock, as well as to prepare solutions for titration. They are using with manual propipetter adjusted by turning the wheel with the thumb or a manual propipetter adjusted by squeezing the bulb.
A graduated pipette is a pipette with its volume, in increments, marked along the tube. It is used to accurately measure and transfer a volume of liquid from one container to another. It is made from plastic or glass tubes and has a tapered tip. Along the body of the tube are graduation markings indicating volume from the tip to that point. A small pipette allows for more precise measurement of fluids; a larger pipette can be used to measure volumes when the accuracy of the measurement is less critical. Accordingly, pipettes vary in volume, with most measuring between 0 and 25.0 millilitres (0.00 and 0.88 imp fl oz; 0.00 and 0.85 US fl oz).
A graduated pipette is a pipette with its volume, in increments, marked along the tube. It is used to accurately measure and transfer a volume of liquid from one container to another. It is made from plastic or glass tubes and has a tapered tip. Along the body of the tube are graduation markings indicating volume from the tip to that point. A small pipette allows for more precise measurement of fluids; a larger pipette can be used to measure volumes when the accuracy of the measurement is less critical. Accordingly, pipettes vary in volume, with most measuring between 0 and 25.0 millilitres (0.00 and 0.88 imp fl oz; 0.00 and 0.85 US fl oz).
Transfer pipettes, also known as Beral pipettes, are similar to Pasteur pipettes, but are made from a single piece of plastic and their bulb can serve as the liquid-holding chamber.
Laboratory flasks.
Laboratory flasks are vessels or containers that fall into the category of laboratory equipment known as glassware. In laboratory and other scientific settings, they are usually referred to simply as flasks. Flasks come in a number of shapes and a wide range of sizes, but a common distinguishing aspect in their shapes is a wider vessel "body" and one (or sometimes more) narrower tubular sections at the top called necks, which have an opening at the top. Laboratory flask sizes are specified by the volume they can hold, typically in metric units such as milliliters (ml) or liters (l). Laboratory flasks have traditionally been made of glass, but can also be made of plastic. At the opening(s) at the top of the neck of some glass flasks such as round-bottom flasks, retorts, or sometimes volumetric flasks, there are outer (or female) tapered (conical) ground glass joints. Some flasks, especially volumetric flasks, come with a laboratory rubber stopper, bung, or cap for capping the opening at the top of the neck. Such stoppers can be made of glass or plastic. Glass stoppers typically have a matching tapered inner (or male) ground glass joint surface, but often only of stopper quality. Flasks which do not come with such stoppers or caps included may be capped with a rubber bung or cork stopper. Flasks can be used for making solutions or for holding, containing, collecting, or sometimes volumetrically measuring chemicals, samples, solutions, etc. for chemical reactions or other processes such as mixing, heating, cooling, dissolving, precipitation, boiling (as in distillation), or analysis.
There are several types of laboratory flasks, all of which have different functions within the laboratory. Flasks, because of their use, can be divided into:
Reaction flasks.
Reaction flasks, which are usually spherical (i.e. round-bottom flask) and are accompanied by their necks, at the ends of which are ground glass joints to quickly and tightly connect to the rest of the apparatus (such as a reflux condenser or dropping funnel). The reaction flask is often made of thick glass and can tolerate large pressure differences, with the result that one can be kept both in a reaction under vacuum, and pressure, sometimes simultaneously. There is at least one tubular section known as the neck with an opening at the tip. Two-, three- or four-necked flasks are common as well. Round bottom flasks come in many sizes, from 5ml to 20l, with the sizes usually inscribed on the glass.
The ends of the necks are typically conical ground glass joints. These are standardized, and can accept any similarly-sized tapered (male) fittings. 24/20 Is common for 250 ml or larger flasks, while smaller sizes such as 14/20 or 19/22 are used for smaller flasks. Because of the round bottom, cork rings are needed to keep the round bottom flasks upright. When in use, round-bottom flasks are commonly held at the neck by clamps on a stand. In pilot plants, even larger flasks are encountered. Some varieties are:
There are several types of laboratory flasks, all of which have different functions within the laboratory. Flasks, because of their use, can be divided into:
Reaction flasks.
Reaction flasks, which are usually spherical (i.e. round-bottom flask) and are accompanied by their necks, at the ends of which are ground glass joints to quickly and tightly connect to the rest of the apparatus (such as a reflux condenser or dropping funnel). The reaction flask is often made of thick glass and can tolerate large pressure differences, with the result that one can be kept both in a reaction under vacuum, and pressure, sometimes simultaneously. There is at least one tubular section known as the neck with an opening at the tip. Two-, three- or four-necked flasks are common as well. Round bottom flasks come in many sizes, from 5ml to 20l, with the sizes usually inscribed on the glass.
The ends of the necks are typically conical ground glass joints. These are standardized, and can accept any similarly-sized tapered (male) fittings. 24/20 Is common for 250 ml or larger flasks, while smaller sizes such as 14/20 or 19/22 are used for smaller flasks. Because of the round bottom, cork rings are needed to keep the round bottom flasks upright. When in use, round-bottom flasks are commonly held at the neck by clamps on a stand. In pilot plants, even larger flasks are encountered. Some varieties are:
- Multiple neck flasks, which can have two to five, and less commonly, six necks, each topped by ground glass connections which are used in more complex reactions that require the controlled mixing of multiple reagents. They are used in synthesis.
- Schlenk flask, which is a spherical flask with a ground glass opening and a hose outlet and a vacuum stopcock. The tap makes it easy to connect the flask to a vacuum-nitrogen line through the hose and to facilitate the carrying out of a reaction either in vacuum or in an atmosphere of nitrogen.
Distillation flasks (Wurtz flasks) are intended to contain mixtures that are subject to distillation, as well as to receive the products of distillation. Distillation flasks are available in various shapes. Similar to the reaction flasks, the distillation flasks usually have only one narrow neck and a ground glass joint and are made of thinner glass than the reaction flask, so that they are easier to heat. They are sometimes spherical, test tube shaped or pear-shaped, also known as Kjeldahl Flasks, due to their use with Kjeldahl bulbs.
The Claisen flasks are generally used for reduced-pressure distillation. The flask was designed to reduce the probability of having to repeat the distillation, due to bumping of the boiling liquid. It is similar to a Würtz flask, though the distinctive feature of the Claisen flask is a U-shaped neck fused on the top of the flask. The flask itself is often round-bottom or pear-shaped. The U-shape (or bifurcation) is similar to that of a Claisen adapter, hence the name. This design makes it impossible for any splash of distilling liquid projected by bumping to reach the distillate.
Round-bottom flasks.
Round-bottom flasks are shaped like a tube emerging from the top of a sphere. The flasks are often long neck; sometimes they have the incision on the neck, which precisely defines the volume of the flask. They can be used in distillations, or in the heating of a product. These types of flask are alternatively called Florence flasks.
Applications:
Round-bottom flasks are shaped like a tube emerging from the top of a sphere. The flasks are often long neck; sometimes they have the incision on the neck, which precisely defines the volume of the flask. They can be used in distillations, or in the heating of a product. These types of flask are alternatively called Florence flasks.
Applications:
- Heating and/or boiling of liquid.
- Distillation.
- Contain chemical reactions.
- Distilling flask in Rotary evaporators.
- Storage of the culture media.
- Preparation of gas-phase standards for flasks fitted with septa (requires volumetric calibration).
The round bottoms on these types of flasks allow more uniform heating and/or boiling of liquid. Thus, round-bottom flasks are used in a variety of applications where the contents are heated or boiled. Round-bottom flasks are used in distillation by chemists as distilling flasks and receiving flasks for the distillate (see distillation diagram). One-neck round-bottom flasks are used as the distilling flasks in rotary evaporators. This flask shape is also more resistant to fracturing under vacuum, as a sphere more evenly distributes stress across its surface.
Round-bottom flasks are often used to contain chemical reactions run by chemists, especially for reflux set-ups and laboratory-scale synthesis. Boiling chips are added to distilling flasks for distillations or boiling chemical reactions to allow a nucleation site for gradual boiling. This nucleation avoids a sudden boiling surge where the contents may overflow from the boiling flask. Stirring bars or other stirring devices suited for round-bottom flasks are sometimes used. Round bottom flasks suffer from poor stirring when compared with Erlenmeyer flasks, as they can't accept large stir bars and material can become trapped at the base. For a reflux set-up, a condenser is typically attached to the middle or only neck of the flask being used. Additional necks on a flask could allow a thermometer or a mechanical stirrer to be inserted into the flask contents. The additional necks can also allow a dropping funnel to be attached to let reactants slowly drip in. Special electrically powered heating mantles are available in various sizes into which the bottoms of round-bottom flasks can fit so that the contents of a flask can be heated for distillation, chemical reactions, boiling, etc. Heating can also be accomplished by submerging the bottom of the flask into a heat bath, water bath, or sand bath. Similarly, cooling can be accomplished by partial submerging into a cooling bath, filled with e.g. cold water, ice, eutectic mixtures, dry ice/solvent mixtures, or liquid nitrogen. For gas preparation where heating is required. Since the flask is round bottomed, heat is uniformly distributed throughout on heating.
Round-bottom flasks are often used to contain chemical reactions run by chemists, especially for reflux set-ups and laboratory-scale synthesis. Boiling chips are added to distilling flasks for distillations or boiling chemical reactions to allow a nucleation site for gradual boiling. This nucleation avoids a sudden boiling surge where the contents may overflow from the boiling flask. Stirring bars or other stirring devices suited for round-bottom flasks are sometimes used. Round bottom flasks suffer from poor stirring when compared with Erlenmeyer flasks, as they can't accept large stir bars and material can become trapped at the base. For a reflux set-up, a condenser is typically attached to the middle or only neck of the flask being used. Additional necks on a flask could allow a thermometer or a mechanical stirrer to be inserted into the flask contents. The additional necks can also allow a dropping funnel to be attached to let reactants slowly drip in. Special electrically powered heating mantles are available in various sizes into which the bottoms of round-bottom flasks can fit so that the contents of a flask can be heated for distillation, chemical reactions, boiling, etc. Heating can also be accomplished by submerging the bottom of the flask into a heat bath, water bath, or sand bath. Similarly, cooling can be accomplished by partial submerging into a cooling bath, filled with e.g. cold water, ice, eutectic mixtures, dry ice/solvent mixtures, or liquid nitrogen. For gas preparation where heating is required. Since the flask is round bottomed, heat is uniformly distributed throughout on heating.
Flasks with flat bottom.
An Erlenmeyer flask, also known as a conical flask or a titration flask, is a type of laboratory flask which features a flat bottom, a conical body, and a cylindrical neck. Erlenmeyer flasks have wide bases, with sides that taper upward to a short vertical neck. They may be graduated, and often spots of ground glass or enamel are used where they can be labeled with a pencil. It differs from the beaker in its tapered body and narrow neck. Depending on the application, they may be constructed from glass or plastic, in a wide range of volumes. The mouth of the Erlenmeyer flask may have a beaded lip that can be stopped or covered. Alternatively, the neck may be fitted with ground glass or other connector for use with more specialized stoppers or attachment to other apparatus. A Büchner flask is a common design modification for filtration under vacuum.
The slanted sides and narrow neck of this flask allow the contents of the flask to be mixed by swirling, without risk of spillage. Such features similarly make the flask suitable for boiling liquids. Hot vapor condenses on the upper section of the Erlenmeyer flask, reducing solvent loss. Erlenmeyer flasks' narrow necks can also support filter funnels. The final two attributes of Erlenmeyer flasks make them especially appropriate for recrystallization. The sample to be purified is heated to a boil, and sufficient solvent is added for complete dissolution. The receiving flask is filled with a small amount of solvent, and heated to a boil. The hot solution is filtered through a fluted filter paper into the receiving flask. Hot vapors from the boiling solvent keep the filter funnel warm, avoiding the premature crystallization. Like beakers, Erlenmeyer flasks are not normally suitable for accurate volumetric measurements. Their stamped volumes are approximate within about 5% accuracy.
The slanted sides and narrow neck of this flask allow the contents of the flask to be mixed by swirling, without risk of spillage. Such features similarly make the flask suitable for boiling liquids. Hot vapor condenses on the upper section of the Erlenmeyer flask, reducing solvent loss. Erlenmeyer flasks' narrow necks can also support filter funnels. The final two attributes of Erlenmeyer flasks make them especially appropriate for recrystallization. The sample to be purified is heated to a boil, and sufficient solvent is added for complete dissolution. The receiving flask is filled with a small amount of solvent, and heated to a boil. The hot solution is filtered through a fluted filter paper into the receiving flask. Hot vapors from the boiling solvent keep the filter funnel warm, avoiding the premature crystallization. Like beakers, Erlenmeyer flasks are not normally suitable for accurate volumetric measurements. Their stamped volumes are approximate within about 5% accuracy.
Büchner flask and funnel.
A Büchner flask, also known as a vacuum flask, filter flask, suction flask, side-arm flask, Kitasato flask or Bunsen flask, is a thick-walled Erlenmeyer flask with a short glass tube and hose barb protruding about an inch from its neck. The short tube and hose barb effectively act as an adapter over which the end of a thick-walled flexible hose (tubing) can be fitted to form a connection to the flask. The other end of the hose can be connected to a source of vacuum such as an aspirator, vacuum pump, or house vacuum. Preferably, this is done through a trap (Wolfe's Flask), which is designed to prevent the sucking back of water from the aspirator into the Büchner flask.
The Büchner flask can also be used as a vacuum trap in a vacuum line to ensure that no fluids are carried over from the aspirator or vacuum pump (or other vacuum source) to the evacuated apparatus or vice versa.
Fritted glass (Schott filter).
Funnels with a Fritted glass named Schott filter, they are used in chemical laboratory practice. Fritted glass is finely porous glass through which gas or liquid may pass. It is made by sintering together glass particles into a solid but porous body. This porous glass body can be called a frit. Applications in laboratory glassware include use in fritted glass filter items, scrubbers, or spargers. Other laboratory applications of fritted glass include packing in chromatography columns and resin beds for special chemical synthesis. Because frits are made up of particles of glass that are bonded together by small contact areas, they are not normally used in strongly alkaline conditions, as these can dissolve the glass to some extent. This is not normally a problem, as the amount dissolved is usually minute, but the equally minute bonds in a frit can be dissolved away by strong alkalis, causing the frit to fall apart over time.
Wolfe's flask.
Wolfe's flask prevents water from entering the vacuum unit in case of sudden "flooding" of the pump due to pressure fluctuations in the water supply system, and also in case of accidental re-throwing of liquids from the plant and prevent them from directly entering the water-jet pump. A hose from the water-jet pump is connected to one branch pipe, and a hose from the plant to the other branch pipe. The ingress of water into the plant is unacceptable for many reasons. In some cases, for example when distilling high boiling liquids under vacuum, this can lead to an explosion.
Funels.
Laboratory funnels are funnels that have been made for use in the chemical laboratory. There are many different kinds of funnels that have been adapted for these specialized applications. Filter funnels, thistle funnels (shaped like thistle flowers), and dropping funnels have stopcocks which allow the fluids to be added to a flask slowly. For solids, a powder funnel with a wide and short stem is more appropriate, as it does not clog easily. When used with filter paper, filter funnels, Buchner and Hirsch funnels can be used to remove fine particles from a liquid in a process called filtration. For more demanding applications, the filter paper in the latter two may be replaced with a sintered glass frit. Separatory funnels are used in liquid-liquid extractions.
Plain funnels exist in various dimensions, with longer or shorter necks. They are used for pour liquids, for separating solids from liquids via the laboratory process of filtering. In order to achieve this, a cone-like shaped piece of filter paper is usually folded into a cone and placed within the funnel. The suspension of solid and liquid is then poured through the funnel. The solid particles are too large to pass through the filter paper and are left on the paper, while the much smaller liquid molecules pass through the paper to a vessel positioned below the funnel, producing a filtrate. The filter paper is used only once. If only the liquid is of interest, the paper is discarded.
Plain funnels exist in various dimensions, with longer or shorter necks. They are used for pour liquids, for separating solids from liquids via the laboratory process of filtering. In order to achieve this, a cone-like shaped piece of filter paper is usually folded into a cone and placed within the funnel. The suspension of solid and liquid is then poured through the funnel. The solid particles are too large to pass through the filter paper and are left on the paper, while the much smaller liquid molecules pass through the paper to a vessel positioned below the funnel, producing a filtrate. The filter paper is used only once. If only the liquid is of interest, the paper is discarded.
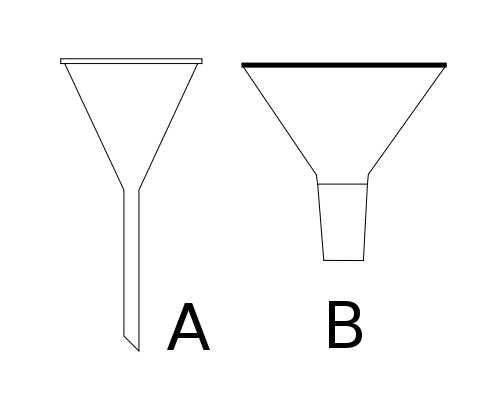
Two funnels, A - a simple stemmed funnel. B - a ground glass powder funnel


Buchner and Hirsch funnels.A Büchner funnel (see above) is a piece of laboratory equipment used in filtration. It is traditionally made of porcelain, but glass and plastic funnels are also available. On top of the funnel-shaped part, there is a cylinder with a fritted glass disc/perforated plate separating it from the funnel. The Hirsch funnel has a similar design; it is used similarly, but for smaller quantities of material. The main difference is that the plate of a Hirsch funnel is much smaller, and the walls of the funnel angle outward instead of being vertical.
A dropping funnel is a type of laboratory glassware used to transfer fluids. They are fitted with a stopcock which allows the flow to be controlled. Dropping funnels are useful for adding reagents slowly, i.e. drop-wise. This may be desirable when the quick addition of the reagent may result in side reactions, or if the reaction is too vigorous.
Dropping funnels are usually fitted with a ground glass joint which allows the funnel to fit snugly onto, e.g. a round bottom flask. This also means it need not be clamped separately. Pressure-equalizing dropping funnels have an additional narrow-bore glass tube from the bulb of the funnel, to the ground glass joint around the stem. These replace the liquid volume lost in the bulb with the equivalent gas volume from the flask into which the reagent is flowing, and are useful when handling air-sensitive reagents in a sealed, inert-gas environment. Without this tube, or some other means to equalize the pressure between a sealed receiving flask and the bulb of the funnel, the flow of fluid from the bulb will rapidly come to a halt.
Dropping funnels are usually fitted with a ground glass joint which allows the funnel to fit snugly onto, e.g. a round bottom flask. This also means it need not be clamped separately. Pressure-equalizing dropping funnels have an additional narrow-bore glass tube from the bulb of the funnel, to the ground glass joint around the stem. These replace the liquid volume lost in the bulb with the equivalent gas volume from the flask into which the reagent is flowing, and are useful when handling air-sensitive reagents in a sealed, inert-gas environment. Without this tube, or some other means to equalize the pressure between a sealed receiving flask and the bulb of the funnel, the flow of fluid from the bulb will rapidly come to a halt.
Note the stopcock, glass tube at the right, and ground glass joint in this pressure-equalizing dropping funnel. An ordinary dropping funnel lacks the pressure-equalizing glass tube on the right side.
Separatory funnels.
A separatory funnel, also known as a separation funnel, separating funnel, or colloquially sep. funnel, is a piece of laboratory glassware used in liquid-liquid extractions to separate (partition) the components of a mixture into two immiscible solvent phases of different densities. Typically, one of the phases will be aqueous, and the other a lipophilic organic solvent such as ether, MTBE, dichloromethane, chloroform, or ethyl acetate. All of these solvents form a clear delineation between the two liquids. The more dense liquid, typically the aqueous phase unless the organic phase is halogenated, sinks and can be drained out through a valve away from the less dense liquid, which remains in the separatory funnel. A separating funnel takes the shape of a cone with a hemispherical end. It has a stopper at the top and stopcock (tap), at the bottom. Separating funnels used in laboratories are typically made from borosilicate glass, and their stopcocks are made from glass or PTFE. Typical sizes are between 30ml and 3l. In industrial chemistry they can be much larger and for much larger volumes centrifuges are used. The sloping sides are designed to facilitate the identification of the layers. The stopcock-controlled outlet is designed to drain the liquid out of the funnel. On top of the funnel there is a standard taper joint which fits with a ground glass or Teflon stopper. To use a separating funnel, the two phases and the mixture to be separated in solution are added through the top with the stopcock at the bottom closed. The funnel is then closed and shaken gently by inverting the funnel multiple times; if the two solutions are mixed together too vigorously, emulsions will form. The funnel is then inverted, and the stopcock carefully opened to release excess vapor pressure. The separating funnel is set aside to allow for the complete separation of the phases. The top and the bottom stopcock are then opened and the lower phase is released by gravitation. The top must be opened while releasing the lower phase to allow pressure equalization between the inside of the funnel and the atmosphere. When the bottom layer has been removed, the stopcock is closed and the upper layer is poured out through the top into another container.
Separating funnel in use. The organic phase (yellow, upper phase) has a lower density than the aqueous phase (green, lower phase). The aqueous phase is being drained into the beaker.
Separating funnel in use. The organic phase (yellow, upper phase) has a lower density than the aqueous phase (green, lower phase). The aqueous phase is being drained into the beaker.
Separating funnels are used in organic chemistry to carry out reactions such as:
- Halogenation,
- Nitration,
- Alkylation,
- Acylation,
- Recovery,
- Organomagnesium synthesis, etc.
Before working with the separating funnel, the valve section is lubricated with petroleum jelly or a special lubricant (vacuum lubricant), which will allow you to open the valve without effort, after which a solution is poured into the funnel itself with the addition (if necessary) of a solvent, with which the reaction flask is pre-rinsed. The amount of liquid in the funnel should not exceed 2/3 of its volume (usually from 1/5 to 1/3), after which it is closed with a stopper and shaken. Further, turning the plug down and fixing it, open the tap. This is necessary so that the airspace of the funnel is saturated with solvent vapors and the pressure in the funnel no longer changes. After the vapor pressure of the solvent becomes constant, and the dissolved gases are removed, it is necessary to vigorously shake the funnel, at the end the funnel is inserted into the rings of the stand and the liquids are allowed to completely separate. After stratification, open the plug and drain the lower layer through the tap, and the upper (if necessary) poured through the throat of the funnel.
Сondensers.
In chemistry, a condenser is laboratory apparatus used to condense vapors — that is, turn them into liquids — by cooling them down. Condensers are routinely used in laboratory operations such as distillation, reflux, and extraction. In distillation, a mixture is heated until the more volatile components boil off, the vapors are condensed, and collected in a separate container. In reflux, a reaction involving volatile liquids is carried out at their boiling point, to speed it up; and the vapors that inevitably come off are condensed and returned to the reaction vessel. In Soxhlet extraction, a hot solvent is infused onto some powdered material, such as ground seeds, to leach out some poorly soluble component; the solvent is then automatically distilled out of the resulting solution, condensed, and infused again. Many different types of condensers have been developed for different applications and processing volumes. The simplest and oldest condenser is just a long tube through which the vapors are directed, with the outside air providing the cooling. More commonly, a condenser has a separate tube or outer chamber through which water (or some other fluid) is circulated, to provide a more effective cooling.
See the Distillation and distillation systems topic for more information.
See the Distillation and distillation systems topic for more information.
A reflux condenser is a laboratory glassware that is used to cool vapors. It consists of a glass tube encased in a glass cylinder. The tube connects the fractioning column with a flask and carries hot vapors produced from heating. Water is contained in the glass cylinder; the water is pumped in and out of the cylinder through its side arms. The water cools the vapor within the tube and condenses it. They are two kinds of reflux condensers. As the vapor condenses, it flows back into the reaction flask. This reduces the amount of solvent that is lost during the reaction. In addition, the reaction can be carried out over an extended period of time, since the solvent is recycled back into the reaction flask. The condenser is mainly used in the distillation process. A distillation is the separation of two liquids by heating. The liquid with the lower boiling point will vaporize first. It is converted back into a liquid inside the condenser. If the condenser deposits the liquid back in the reaction flask, it is called a reflux condenser. There are two types of reflux condensers: air cooled and water cooled. The common air cooled reflux condensers include the air condenser and the Vigreux condenser. A Liebig condenser is the simplest water cooled reflux condenser. The Dimroth condenser and the Graham condenser are two other water cooled reflux condensers. The air cooled reflux condenser only has one glass tube, and the vapors condense on the glass as they are cooled by the air. Some air cooled reflux condensers are filled with glass beads to aid in the condensation process. The Vigreux condenser features a series of indentations designed to increase the amount of surface area available for the vapor to condense on. Water cooled reflux condenser has two glass tubes. The inner tube carries the hot vapor, while the outer tube carries the water. Water is used to cool the vapor. The Liebig condenser features a straight inner tube, while the Graham condenser has a spiral inner tube. There is a double spiral tube within the Dimroth condenser.
The Soxhlet extractor.
The Soxhlet extractor is used for liquid-solid extractions when the compound to be extracted has limited solubility in the chosen solvent and the impurities are insoluble.
During the extraction, solvent vapour will flow up the distillation path, into the main chamber and up into the condenser where it will condense and drip down. The solvent will fill the main chamber, dissolving some of the desired compound from the solid sample. Once the chamber is almost full, it is emptied by the siphon, returning the solvent to the round bottom flask to begin the process again. Each time the extraction is repeated, more of the desired compound is dissolved, leaving the insoluble impurities in the thimble. This is how a compound is removed from the sample.
During the extraction, solvent vapour will flow up the distillation path, into the main chamber and up into the condenser where it will condense and drip down. The solvent will fill the main chamber, dissolving some of the desired compound from the solid sample. Once the chamber is almost full, it is emptied by the siphon, returning the solvent to the round bottom flask to begin the process again. Each time the extraction is repeated, more of the desired compound is dissolved, leaving the insoluble impurities in the thimble. This is how a compound is removed from the sample.
1: Stirrer bar 2: Still pot (the still pot should not be overfilled and the volume of solvent in the still pot should be 3 to 4 times the volume of the soxhlet chamber) 3: Distillation path 4: Thimble 5: Solid 6: Siphon top 7: Siphon exit 8: Expansion adapter 9: Condenser 10: Cooling water out 11: Cooling water in
Unlike a traditional extraction method, a small amount of solvent is reused to perform an extraction many times. This means that much less solvent is used in a Soxhlet extraction, making it more time and cost-effective. Also, the Soxhlet extractor can run continuously without any further operation, making it an excellent choice for extracting compounds over hours or even days.
Franz Ritter von Soxhlet first invented the apparatus to extract lipids (fats) from milk solids. Now, the Soxhlet extractor is used whenever exhaustive extractions are needed, particularly in the oil and food industries. It is also widely used for extracting bioactive compounds from natural resources, which is crucial in environmental analysis of soils and wastes.
How to use it?
- The Soxhlet extractor will run continuously once set up correctly:
- Load the sample material containing the desired compound into the thimble.
- Place the thimble into the main chamber of the Soxhlet extractor.
- Add the chosen solvent to a round bottom flask and place onto a heating mantle.
- Attach the Soxhlet extractor above the round bottom flask.
- Attach a reflux condenser above the extractor, with cold water entering at the bottom and exiting above.
- Now the apparatus is set up, heat the solvent to reflux and leave to extract for the required amount of time.
Ground glass joints and adapters.
This type of glassware, commonly known as Quickfit, comprises a complete range of components fitted with standard-taper ground-glass joints. The joints are fully interchangeable with those of the same size and apparatus for a whole range of experiments can be assembled from the simple components without the need to use rubber bungs, corks, etc. Where there is a mismatch between the sizes of the joints of the pieces of glassware, reduction and expansion adapters can be used. A typical range of jointed glassware is illustrated in pictures below.
The ground-glass joint on the glassware is classified according to the diameter of the joint at its widest point (internal diameter) and the length of the ground-glass portion of the joint. Thus, a 14/23 joint has a maximum internal diameter of 14 mm and a length of 23 mm. Other common joint sizes you will frequently encounter are 19/26, 24/29 and 35/39. The joint size is always etched into glass on the side of or near to the joint. For obvious reasons, joints are categorized as 'female' and 'male'.
Jointed glassware is much more expensive than ordinary glassware because of the precision required in fabricating the joints. If the joints 'seize' and cannot be separated the glassware cannot be used again, and you may have the problem of a stoppered flask containing a volatile organic solvent, which somebody has to open!
There are two main causes of "seized" joints:
There are two main causes of "seized" joints:
- Using solutions of potassium hydroxide or sodium hydroxide in water or other solvents, which attack the glass.
- Trapping chemicals, including solids and solutions of solids, in the ground-glass joints.
If you are using jointed glassware with strong alkalis (NaOH, KOH), you must grease the joints. In most cases, a simple hydrocarbon-based grease, such as petroleum jelly, will suffice, since it is easily removed from the joints by wiping with a cloth wet with a hydrocarbon solvent (petroleum spirit, b.pt. 60- 80 °C). Avoid silicone-based grease, since this is difficult to remove, soluble in some organic solvents and may contaminate your reaction products. To grease a joint, put a small smear of grease on the upper part of the 'male' joint, push it into the 'female' joint with a twisting movement and the joint should become 'clear' from the top to about half-way down. If more than half the joint has become 'clear', you have used too much grease: separate the joints, clean with a solvent-soaked cloth and repeat the process. To avoid trapping chemicals in the ground-glass joints, fill flasks etc. using a long-stemmed filter funnel or paper cone, which extends past the joint into the flask.
The Claisen adapter.
The Claisen adapter can be placed on top of a round bottom flask to convert one opening into two, For example, attach one upper joint of the Claisen adapter to a condenser and one to an additional funnel or accept a thermometer adapter for temperature measurements in a distillation apparatus; This Claisen adapter has two upper outer joint to attach any lab glassware with inner joints, and a lower inner joint to entry into a boiling flask with outer joint. The sizes of the three joints are the same 24/40. Labor Glass Claisen adapter is made of high-quality borosilicate glass and annealed at 800 degree Celsius, can be heated directly in an open flame and can withstand typical laboratory thermal variations in chemistry processes like heating and cooling.
The Claisen adapter.
The Claisen adapter can be placed on top of a round bottom flask to convert one opening into two, For example, attach one upper joint of the Claisen adapter to a condenser and one to an additional funnel or accept a thermometer adapter for temperature measurements in a distillation apparatus; This Claisen adapter has two upper outer joint to attach any lab glassware with inner joints, and a lower inner joint to entry into a boiling flask with outer joint. The sizes of the three joints are the same 24/40. Labor Glass Claisen adapter is made of high-quality borosilicate glass and annealed at 800 degree Celsius, can be heated directly in an open flame and can withstand typical laboratory thermal variations in chemistry processes like heating and cooling.
Design.
The Claisen adapter can be placed on top of a round bottom flask to convert one opening into two, For example, attach one upper joint of the Claisen adapter to a condenser and one to an additional funnel or accept a thermometer adapter for temperature measurements in a distillation apparatus; This Claisen adapter has two upper outer joint to attach any lab glassware with inner joints, and a lower inner joint to entry into a boiling flask with outer joint.
USE.
Used in situations which require more than one outlet from a round-bottom flask, ideal for a reflux of a reaction mixture, one joint fit a glass condenser, one fit an additional funnel. In practice, it is causally used in a distillation apparatus and placed on the distillation flask, the extra neck can be used to add water into the boiling flask during distillation process.
The 3 way Claisen adapter is with three 24/40 standard taper joints for quickly and easily fit leak-tight lab glassware. The top two joints are female to attach the distillation head and an addition funnel or powder funnel.
The Claisen adapter can be placed on top of a round bottom flask to convert one opening into two, For example, attach one upper joint of the Claisen adapter to a condenser and one to an additional funnel or accept a thermometer adapter for temperature measurements in a distillation apparatus; This Claisen adapter has two upper outer joint to attach any lab glassware with inner joints, and a lower inner joint to entry into a boiling flask with outer joint.
USE.
Used in situations which require more than one outlet from a round-bottom flask, ideal for a reflux of a reaction mixture, one joint fit a glass condenser, one fit an additional funnel. In practice, it is causally used in a distillation apparatus and placed on the distillation flask, the extra neck can be used to add water into the boiling flask during distillation process.
The 3 way Claisen adapter is with three 24/40 standard taper joints for quickly and easily fit leak-tight lab glassware. The top two joints are female to attach the distillation head and an addition funnel or powder funnel.
Bubblers.
Bubblers are simple devices, used to maintain an inert atmosphere over a reaction apparatus, while also providing for a means for pressure relief. Bubblers are typically filled with mercury or mineral oil, however the latter is recommended because mercury bubblers splash quite a bit and pose a toxicity hazard.
When the pressure inside your apparatus is greater than the laboratory's atmospheric pressure, excess gas will bubble down the tube and out through the mineral oil. If the pressure inside your apparatus falls below atmospheric pressure, oil will rise in the tube and prevent air from entering the system. However, if the pressure is too low, air will eventually get in, and you will suck oil (or mercury) into your apparatus. This is the kind of mistake you generally make only once or twice (the tedious cleanup is a great learning experience).
You can avoid having your bubbler "suck back" by:
- Being careful not to induce negative pressure in your system while it is open to the bubbler. The three most common causes of this are:
- Pulling a vacuum on the flask when it is open to the bubbler.
- Turning off the heat on a hot reaction, but not increasing the nitrogen flow.
- Cooling your reaction in a cold bath, but not increasing the nitrogen flow.
- Using specially modified bubblers.
- Using a mercury bubbler that is taller than 760 mm (the maximum height that mercury can achieve with 1 atm of pressure).
The tube between the bubbler and the reactor has to have a higher temperature than the bubble, otherwise the precursor would condense in the tube and therefore uncontrolled droplets would be passed into the reaction vessel. If this happens with a solid precursor, it could plug the line. If you bubble something other than nitrogen (HCl, solvents, reaction byproducts) through your bubbler be sure to either bubble pure nitrogen through it when you are done or clean the bubbler. This way, you'll avoid contaminating your next reaction.
Note: Be sure your bubbler liquid does not react with the gases you are using. For example, mercury is incompatible with ammonia and acetylene.
To reduce the chance of accidental pressure explosions, NEVER open a gas cylinder to a vacuum manifold unless the manifold is open to a bubbler!
To maintain a positive pressure on a reaction that is simply stirring, the bubbler should bubble once every few seconds. A greater flow wastes nitrogen and can bubble away volatile solvents. A lesser flow increases the chances of air diffusing into your apparatus. To prevent oil or mercury from splashing out of your bubbler, connect a piece of Tygon tubing to the outlet. Arrange this vertically several inches, or make several coils in the tubing. Alternatively, you can attach an empty bubbler to your bubbler outlet to retain any splashed material.
Avoid using mercury in the laboratory whenever possible. But if you must use it, be sure to read these tips, warnings and guidelines.
Safety Considerations.
Common Causes of Explosion:
Note: Be sure your bubbler liquid does not react with the gases you are using. For example, mercury is incompatible with ammonia and acetylene.
To reduce the chance of accidental pressure explosions, NEVER open a gas cylinder to a vacuum manifold unless the manifold is open to a bubbler!
To maintain a positive pressure on a reaction that is simply stirring, the bubbler should bubble once every few seconds. A greater flow wastes nitrogen and can bubble away volatile solvents. A lesser flow increases the chances of air diffusing into your apparatus. To prevent oil or mercury from splashing out of your bubbler, connect a piece of Tygon tubing to the outlet. Arrange this vertically several inches, or make several coils in the tubing. Alternatively, you can attach an empty bubbler to your bubbler outlet to retain any splashed material.
Avoid using mercury in the laboratory whenever possible. But if you must use it, be sure to read these tips, warnings and guidelines.
Safety Considerations.
Common Causes of Explosion:
- Use of pressurized gases – Explosion can occur if the inert gas pressure builds up in a closed system. Make sure there is a source of pressure relief in the form of a bubbler and that there is not a closed system when the gas line is open. An electronic pressure gauge or manometer can also be added to the line to monitor the pressure and provide extra peace of mind.
- Out of control reaction – A violent reaction can evolve a large volume of gas quickly. Again, ensure there is adequate pressure relief in the system, i.e., a bubbler, and that the reaction vessel is open to the line.
- Heating a closed system – Increasing the temperature of a closed system (constant volume) increases the pressure. Make sure any vessel you heat is open to the line and there is pressure relief in the form of a bubbler, attached to the line.
Common Cause of Implosion:
- Cracks in glassware – Any weakness in the glassware, such as a star crack, can cause it to fail under vacuum. If you notice a crack in a vessel, do not use it.
Conclusion:
I hope my description and short manuals will help you to reach your goals. If you need some extra explanation, you can ask me there or private chat. I will add some information as needed. You must always think about safety during work with a glassware in laboratory. Use safety glass, chemical coat, gloves to prevent injuries and chemical burns, accidents with eyes.
Last edited by a moderator:

