G.Patton
Expert
- Joined
- Jul 5, 2021
- Messages
- 2,412
- Solutions
- 3
- Reaction score
- 2,382
- Points
- 113
- Deals
- 1
Introduction
In the synthesis of mephedrone (4-MMC) and other psychoactive substances at different stages (synthesis and purification) use different solvents, which are not entered into a chemical reaction. They remain in the same form as before syntheses. However, they are mixed with dirt, and their use without significant cleaning (regeneration) in the upcoming syntheses is impossible. The share of these solvents in the waste is about 50%. Almost all of these solvents can be restored and reused. This is a fairly large share of the product cost, and regeneration can reduce the risks:
- Some solvents are controlled by governments (acetone, for example) and it is better not to buy them once again. Those, that are not controlled, are also not worth buying again.
- Waste reduction. Every 5 kg of mephedrone generate up to ~ 75 kg of waste (actually more with water), reducing this figure by half also gives a safety plus.
In general, if you are seriously into mephedrone synthesis, you need to keep this topic in mind. We will talk here about the regeneration of those solvents that we use, namely:
1. Isopropyl alcohol (IPA)
2. Dichloromethane (DCM)
3. Acetone
4. Ortho-xylene (less harmless replacement for benzene and toluene)
5. Diethyl ether
6. Benzene
It will not be possible to regenerate 100% of solvents. But reducing purchases by 10 times (if 90% is regenerated) is also a very correct and practical task.
1. IPA
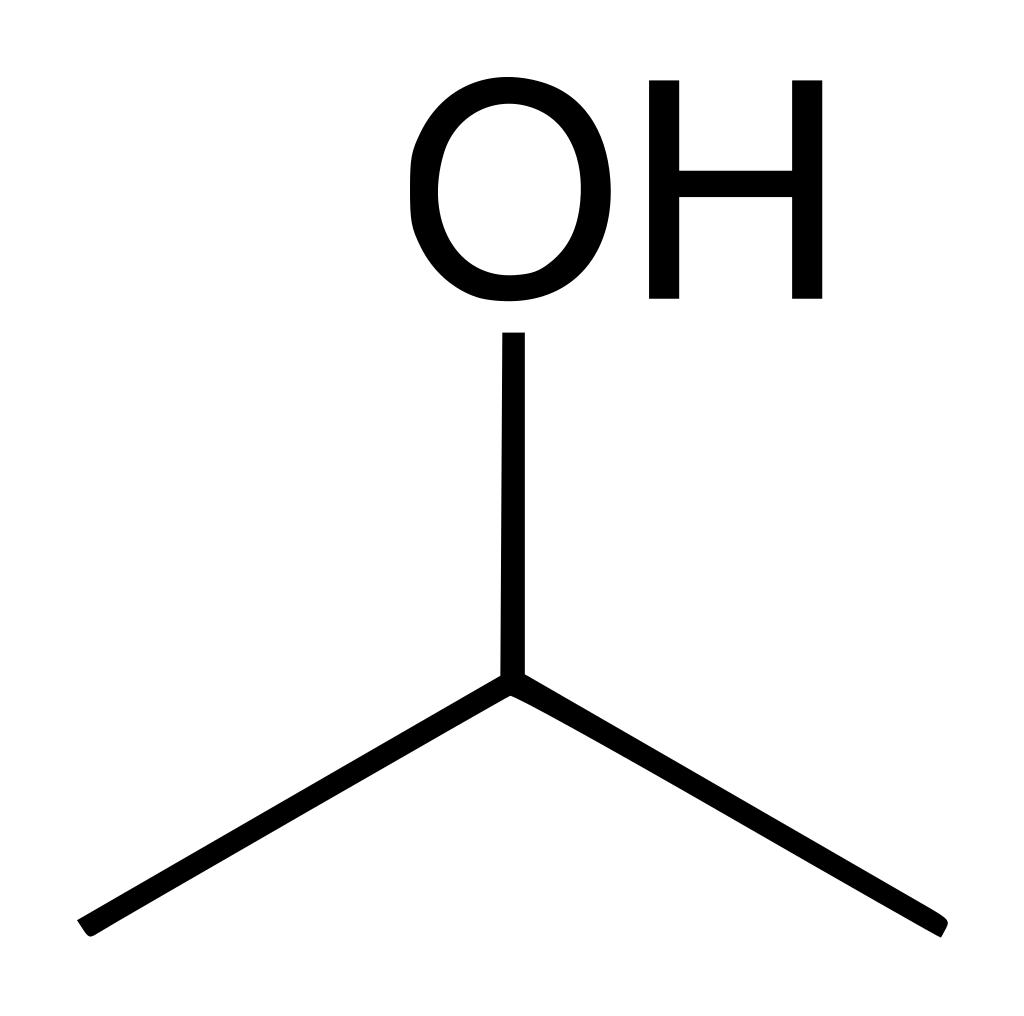
A large amount of IPA waste is generated as a result of product purification using IPA. IPA is also used to wash a solid product. For example, after solidification, when the use of acetone is not very good since it reacts with the remaining acid, coloring the product in all colors of the rainbow. After purification of mephedrone, IPA comes mainly in the form of a mixture with water (which IPA helps to expel from the mixture).
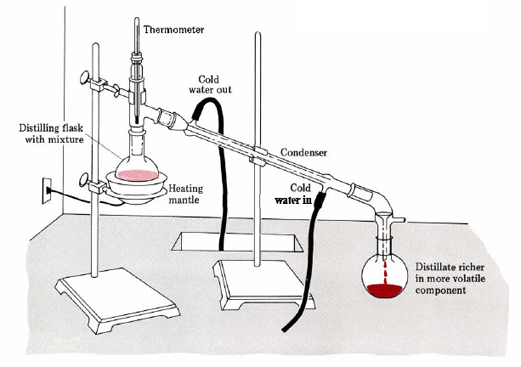
Firstly, I recommend to distillate IPA, boiling point 82.5 °C. If you don't need very pure IPA, or you're certain that it isn't contain pollution, you can do just the following actions without distillation.
This water-IPA mixture is regenerated very simply: calcium chlorides (anhydrous) are poured in, which is sold in any chemical store without problems, at the rate of 1 kg of CaCl2 per 10 liters of the mixture and mixed well (can be done by shaking). Close the vessel with IPA tightly and put it in the freezer (for the night) because taking water from the IPA is better in the cold. After 6-8 h, the mixture is removed from the freezer, while calcium chloride "freezes" into a dense mass. Dry IPA is filtered from the sediment. The last 5-10 % of IPA are cloudy (containing CaCl2 suspension), the solution can either be carefully drained or filtered on a funnel through a regular paper filter. The impurities that are found in this IPA (such as DCM residue or acetone) do not affect the performance of the IPA in further cleanses. Considering that IPA is consumed more than all other solvents (up to 33 liters per 5 kg of mephedrone), this is the most effective recovery.
Firstly, I recommend to distillate IPA, boiling point 82.5 °C. If you don't need very pure IPA, or you're certain that it isn't contain pollution, you can do just the following actions without distillation.
This water-IPA mixture is regenerated very simply: calcium chlorides (anhydrous) are poured in, which is sold in any chemical store without problems, at the rate of 1 kg of CaCl2 per 10 liters of the mixture and mixed well (can be done by shaking). Close the vessel with IPA tightly and put it in the freezer (for the night) because taking water from the IPA is better in the cold. After 6-8 h, the mixture is removed from the freezer, while calcium chloride "freezes" into a dense mass. Dry IPA is filtered from the sediment. The last 5-10 % of IPA are cloudy (containing CaCl2 suspension), the solution can either be carefully drained or filtered on a funnel through a regular paper filter. The impurities that are found in this IPA (such as DCM residue or acetone) do not affect the performance of the IPA in further cleanses. Considering that IPA is consumed more than all other solvents (up to 33 liters per 5 kg of mephedrone), this is the most effective recovery.
2. DCM
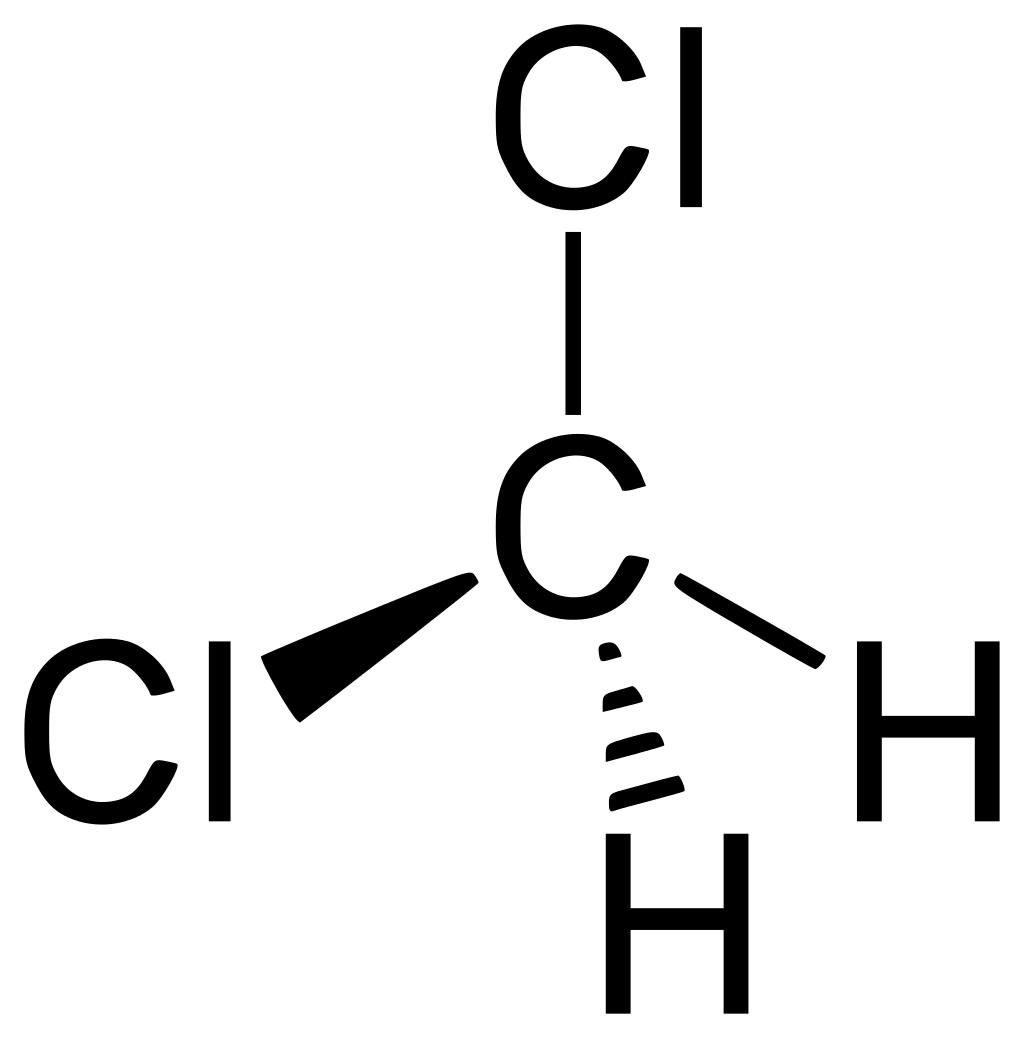
Restoring DCM (CH2Cl2) is more difficult by reason of the hard safety measures. The fact is that during the distillation of DCM with water, DCM is partially oxidized by atmospheric oxygen, forming a rather toxic gas - formaldehyde. If anyone doesn't know, this is the main "damaging factor" of methyl alcohol, which decomposes in a body to this compound, which is responsible for all poisoning. So, the work with distilling of DCM must be carried out strictly in a closed system, the exit from the condenser must be made directly into the hood or pull out probe with good suction speed.
Contaminated DCM is poured into a distillation flask and boiled. DCM boils at 40 °C and its azetrope with water at 38 °C. It boils violently, with "explosions", so pour no more than a half or 1/3 volume of rb flask. I also advise you to use boiling chips, such as broken porcelain cups, broken tiles. As the mixture evaporates, the temperature of the mixture rises very fast, new portions of dirty DCM are added, pollutions are concentrated, about 1/10 of the DCM remains with this dirt in the evaporation flask. Next, it is drained, and the flask, in a gas mask (!), is washed with some remnants of acetone or/and IPA, plums are utilized. Thus, up to 80-90 % of DCM is obtained, which is a rather hard-to-get solvent, and even heavy (1.3 kg per liter).
The resulting secondary DСM is gray, sometimes even yellowish, which does not interfere with its use again. It contains water, which also does not interfere because all processes involving DСM also include aqueous solutions. DCM must be washed after distillation from the remnants of IPA (this happens) and formaldehyde.
It is done like this: DCM is poured into the flask (reactor), the same amount of distilled water, the mixture is stirred, the solution is divided into layers, the DCM layer is drained. The same water can be used to wash 3-4 batches of DCM, formaldehyde and IPA remains are perfectly soluble in water, and during separation remain in the water layer, which is discarded after all washes. And DCM, which accounts for up to 1/3 of the weight of hard-to-find reagents, is ready to go again.
If you get a mixture of DСM, IPA, water, and pollutions as a result of acidification in DСM and IPA, then only DСM will come out properly. To accomplish this, the mixture is filled with water, approximately 70-80% of the total volume of the mixture. Then it separates, leaving the DСM with "own" dirt alone (well, almost with traces of IPA), and IPA, water and water-soluble dirt are separated. The DСM is then distilled as above and washed 2-3 times with water to remove remains of IPA that will interfere with further DCM use. It is possible to extract IPA from Water with IPA (about 30% IPA) solution by several successive distillations (2-3 times), consistently enriching the IPA percentage content. At the same time, a significant part of the IPA is lost, even if you decide to get confused with such a distillation. DСM is a much more valuable reagent, and it makes sense to isolate it even with such a somewhat more complicated procedure. In addition, IPA with water can be drained into the sewerage, while DСM is highly discouraged because separating from the water in the sewerage, it (as a heavier immiscible liquid) accumulates in some cavities; DCM corrodes plastic and rubber, this action can lead to accidents in the sewers, which can indicate the location of your laboratory. Simply put, either regenerate it or pour it into canisters and utilize.
Contaminated DCM is poured into a distillation flask and boiled. DCM boils at 40 °C and its azetrope with water at 38 °C. It boils violently, with "explosions", so pour no more than a half or 1/3 volume of rb flask. I also advise you to use boiling chips, such as broken porcelain cups, broken tiles. As the mixture evaporates, the temperature of the mixture rises very fast, new portions of dirty DCM are added, pollutions are concentrated, about 1/10 of the DCM remains with this dirt in the evaporation flask. Next, it is drained, and the flask, in a gas mask (!), is washed with some remnants of acetone or/and IPA, plums are utilized. Thus, up to 80-90 % of DCM is obtained, which is a rather hard-to-get solvent, and even heavy (1.3 kg per liter).
The resulting secondary DСM is gray, sometimes even yellowish, which does not interfere with its use again. It contains water, which also does not interfere because all processes involving DСM also include aqueous solutions. DCM must be washed after distillation from the remnants of IPA (this happens) and formaldehyde.
It is done like this: DCM is poured into the flask (reactor), the same amount of distilled water, the mixture is stirred, the solution is divided into layers, the DCM layer is drained. The same water can be used to wash 3-4 batches of DCM, formaldehyde and IPA remains are perfectly soluble in water, and during separation remain in the water layer, which is discarded after all washes. And DCM, which accounts for up to 1/3 of the weight of hard-to-find reagents, is ready to go again.
If you get a mixture of DСM, IPA, water, and pollutions as a result of acidification in DСM and IPA, then only DСM will come out properly. To accomplish this, the mixture is filled with water, approximately 70-80% of the total volume of the mixture. Then it separates, leaving the DСM with "own" dirt alone (well, almost with traces of IPA), and IPA, water and water-soluble dirt are separated. The DСM is then distilled as above and washed 2-3 times with water to remove remains of IPA that will interfere with further DCM use. It is possible to extract IPA from Water with IPA (about 30% IPA) solution by several successive distillations (2-3 times), consistently enriching the IPA percentage content. At the same time, a significant part of the IPA is lost, even if you decide to get confused with such a distillation. DСM is a much more valuable reagent, and it makes sense to isolate it even with such a somewhat more complicated procedure. In addition, IPA with water can be drained into the sewerage, while DСM is highly discouraged because separating from the water in the sewerage, it (as a heavier immiscible liquid) accumulates in some cavities; DCM corrodes plastic and rubber, this action can lead to accidents in the sewers, which can indicate the location of your laboratory. Simply put, either regenerate it or pour it into canisters and utilize.
3. Acetone
Acetone is a very capricious solvent for regeneration. The water, which is dissolved with acetone, is removed with great difficulty. It is easier with organic pollutions, acetone is purified by distillation as DCM. If there are some other solvents are dissolved in acetone, it is evaporated from the polluted solution without water. Also, as the next portions of dirty acetone are added, the temperature of the mixture rises, and at the temperature more than 75-80 °C, evaporation should be stopped, leaving a little acetone with the dirty residue. It is not necessary to dry and rinse the distilled acetone.
The problem is that this way help to regenerate acetone only 2-3 times (according to experience). Acetone is used as washing solvent for wet mephedrone with remnants of IPA or DCM, for example. These solvents are evaporated together with acetone their boiling points are close, and they evaporate with water (unlike acetone), which is can't be removed from this mixture by any calcium chloride or another dry agent. After 2-3 distillations, such acetone-IPA is disposed of for glassware washing purposes. It is possible to extend the life of secondary acetone by drying mephedrone before washing with acetone. This procedure is not optimal by reason of time-consuming. Mephedrone dries for quite a long time after cleaning with IPA, especially in case of not very clean product.
Small amounts of IPA do not prevent acetone from washing a product, especially in ice form. Water can be removed by helps of distillation over phosphorus pentoxide P2O5; Dry the acetone with anhydrous potash (about 5% by weight of acetone) is heated for several hours with reflux, pour into another flask and distill over fresh drying agent; The boiling point of acetone is 56.2 °C. Metallic sodium and alkalis are unsuitable for drying acetone
The problem is that this way help to regenerate acetone only 2-3 times (according to experience). Acetone is used as washing solvent for wet mephedrone with remnants of IPA or DCM, for example. These solvents are evaporated together with acetone their boiling points are close, and they evaporate with water (unlike acetone), which is can't be removed from this mixture by any calcium chloride or another dry agent. After 2-3 distillations, such acetone-IPA is disposed of for glassware washing purposes. It is possible to extend the life of secondary acetone by drying mephedrone before washing with acetone. This procedure is not optimal by reason of time-consuming. Mephedrone dries for quite a long time after cleaning with IPA, especially in case of not very clean product.
Small amounts of IPA do not prevent acetone from washing a product, especially in ice form. Water can be removed by helps of distillation over phosphorus pentoxide P2O5; Dry the acetone with anhydrous potash (about 5% by weight of acetone) is heated for several hours with reflux, pour into another flask and distill over fresh drying agent; The boiling point of acetone is 56.2 °C. Metallic sodium and alkalis are unsuitable for drying acetone
4. Ortho-Xylene
Ortho-Xylene is an undeservedly "forgotten" solvent. It has many valuable properties. It is not as toxic, carcinogenic, and not as volatile (bp 144 °C) as benzene or toluene. Synthesis time and temperature values are similar with benzene solvent (already checked). O-xylene, almost immiscible with water (0.014%). The azeotropic mixture of o-xylene with water boils at 92 °C and contains 64.25% o-xylene and 35.75% water. Thus, xylene regeneration looks like this:
2/3 Xylene and 1/3 distilled water are poured into the round bottom flask [fill half volume with the flask]. The mixture boils, as it should be for a high-boiling solvent, slowly and gradually, the water from the low layer gives steam jets that pass through the top layer of xylene. This forms a cap of foam, which can suck dirt into the reflux condenser. You should pour a half volume to the of the flask and add boiling chips to prevent foam formation. Two layers are formed at once in the receiving flask, the lower layer is water (poured out), xylene is additionally washed with water for purification because some pollutants enters into the receiving flask (apparently due to the high boiling point of the azeotrope). Xylene is almost immiscible with water, so it is not necessary to dry the regenerated product.
5. Diethyl ether
Extremely flammable; Vapors form explosive mixtures with air. Vapors are approximately 2.6 times heavier than air and can spread over the surface of the worktable. Therefore, it is necessary to ensure that in the vicinity (up to 1 m) from the place of work with ether, all gas burners are extinguished, and electric stoves with an open spiral are disconnected from the mains. During storage of diethyl ether under the action of light and atmospheric oxygen, explosive peroxide compounds and acetaldehyde are formed in it. Peroxy compounds are the cause of extremely violent explosions, especially when attempting to distill ether to dryness. Many reactions have been proposed for the detection of peroxide in diethyl ether. The ether is washed with 5% NaOH solution and water, dried for 24 h over anhydrous CaCl2 (150-200 g CaCl2 per 1 liter of ether). The CaCl2 is then filtered off on a large filter paper and the ether is collected in a dark glass bottle. The flask is tightly closed with a cork stopper with a calcium chloride tube filled with CaCl2, bent at an acute angle, inserted into it. Then, having opened the flask, sodium wire is briefly introduced into the ether, at the rate of 5 g per 1 liter of ether.
After 24 hours, when no more hydrogen bubbles are emitted, another 3 g of sodium wire per 1 liter of ether is added, and after 12 hours the ether is poured into a distillation flask and distilled over sodium wire. The receiver must be protected by a calcium chloride tube with CaCl2. The distillate (boiling point 34.6 °C)is collected in a dark glass bottle, which, after adding 1 g of sodium wire per 1 liter of ether, is closed with a cork stopper with a calcium chloride tube and stored in a cold and dark place. If the surface of the wire has changed greatly and hydrogen bubbles are released again when the wire is added, then the ether should be filtered into another flask and another portion of sodium wire should be added.
A convenient and very effective way to purify diethyl ether from peroxides and at the same time from moisture is to pass the ether through a column with active Al2O3. Columns with a height of 60-80 cm and a diameter of 2-4 cm, filled with 82 g of Al2O3, are sufficient to purify 700 ml of ether containing a significant amount of peroxide compounds. Waste Al2O3 can be easily regenerated if a 50% acidified aqueous solution of FeSO4. 7H2O is passed through the column, washed with water, dried, and thermally activated at 400-450 °C.
Absolute ether is a highly hygroscopic liquid. The degree of moisture absorption by ether during its storage can be determined by the blueness of the anhydrous white CuSO4 powder when it is introduced into ether (a colored hydrate CuSO4.5H2O is formed).
After 24 hours, when no more hydrogen bubbles are emitted, another 3 g of sodium wire per 1 liter of ether is added, and after 12 hours the ether is poured into a distillation flask and distilled over sodium wire. The receiver must be protected by a calcium chloride tube with CaCl2. The distillate (boiling point 34.6 °C)is collected in a dark glass bottle, which, after adding 1 g of sodium wire per 1 liter of ether, is closed with a cork stopper with a calcium chloride tube and stored in a cold and dark place. If the surface of the wire has changed greatly and hydrogen bubbles are released again when the wire is added, then the ether should be filtered into another flask and another portion of sodium wire should be added.
A convenient and very effective way to purify diethyl ether from peroxides and at the same time from moisture is to pass the ether through a column with active Al2O3. Columns with a height of 60-80 cm and a diameter of 2-4 cm, filled with 82 g of Al2O3, are sufficient to purify 700 ml of ether containing a significant amount of peroxide compounds. Waste Al2O3 can be easily regenerated if a 50% acidified aqueous solution of FeSO4. 7H2O is passed through the column, washed with water, dried, and thermally activated at 400-450 °C.
Absolute ether is a highly hygroscopic liquid. The degree of moisture absorption by ether during its storage can be determined by the blueness of the anhydrous white CuSO4 powder when it is introduced into ether (a colored hydrate CuSO4.5H2O is formed).
6. Benzene
Benzene and its homologues, toluene and xylenes, are widely used as solvents and azeotropic drying media. Benzene should be handled with special safety equipment due to its flammability and toxicity, as well as the formation of explosive mixtures with air. Benzene vapor with repeated exposure disrupts the normal function of the hematopoietic organs; in the liquid state, benzene is strongly absorbed through the skin and irritates it. Benzene forms an azeotropic mixture with water (8.83 wt %, bp 69.25 °C). Therefore, before distillation, wet benzene is boiled with a Dean-Stark apparatus and the water is almost completely distilled off. Additional drying of distilled benzene is usually carried out with calcined CaCl2 (for 2-3 days) and sodium wire. During the distillation, care must be taken to ensure that the distilled benzene does not crystallize in a condenser (Tm 5.5 °C).
Technical benzene contains up to 0.05% wt. thiophene, which cannot be separated from benzene either by fractional distillation or crystallization (freezing). Thiophene in benzene is detected as follows: a solution of 10 mg of isatin in 10 ml of conc. H2SO4 is shaken with 3 ml of benzene. In the presence of thiophene, the sulfuric acid layer turns blue-green. Benzene is purified from thiophene by repeated extraction with concentrated H2SO4. For 1 liter of benzene, take 80 ml of acid. Purification is carried out until a faint yellow color of the acid is achieved. After separation of the acid layer, the benzene is washed with water, then with a 10% Na2CO3 solution and again with water, after which the benzene is distilled. A more efficient and simple method for removing thiophene from benzene is boiling 1 liter of benzene with 100 g of Raney nickel in a flask under reflux for 15-30 minutes. Another way to purify benzene from thiophene is to fractionally crystallize it from ethyl alcohol. A saturated solution of benzene in alcohol is cooled to about -15 °C, solid benzene is quickly filtered off and distilled.
Technical benzene contains up to 0.05% wt. thiophene, which cannot be separated from benzene either by fractional distillation or crystallization (freezing). Thiophene in benzene is detected as follows: a solution of 10 mg of isatin in 10 ml of conc. H2SO4 is shaken with 3 ml of benzene. In the presence of thiophene, the sulfuric acid layer turns blue-green. Benzene is purified from thiophene by repeated extraction with concentrated H2SO4. For 1 liter of benzene, take 80 ml of acid. Purification is carried out until a faint yellow color of the acid is achieved. After separation of the acid layer, the benzene is washed with water, then with a 10% Na2CO3 solution and again with water, after which the benzene is distilled. A more efficient and simple method for removing thiophene from benzene is boiling 1 liter of benzene with 100 g of Raney nickel in a flask under reflux for 15-30 minutes. Another way to purify benzene from thiophene is to fractionally crystallize it from ethyl alcohol. A saturated solution of benzene in alcohol is cooled to about -15 °C, solid benzene is quickly filtered off and distilled.
Benzene from sodium benzoate
Conclusion
The recovering of each individual solvent is economically beneficial. Separating of mixtures of different solvents by fractions is much more complicated. For successful regeneration, it is better to choose synthesis ways to use solvents that do not mix them with each other. Currently, the process which ends with acidification with hydrochloric acid with the selection and washing of the aqueous fraction looks like the most sensible one. The first product solution cleaning uses DCM, then you have to use boiling with IPA, then washing the final product with acetone. In conclusion, cleaning with three different solvents that do not mix with each other during use (especially if the product is dried after cleaning in IPA) ensures good product purity and the possibility of recovering a significant part of the solvents.
Last edited by a moderator: