G.Patton
Expert
- Joined
- Jul 5, 2021
- Messages
- 2,412
- Solutions
- 3
- Reaction score
- 2,381
- Points
- 113
- Deals
- 1
Introduction

I want to show in this topic simple rules of handling and video manual of liquid nitrogen dewar bath with acetone preparing. This thread is the next part of low-temperature bath topics, you can learn previous part Dry ice (-78.5 deg) laboratory handling to be more confident and safe in low-temperature synthesis procedures.
General
Cryogenic liquids have boiling points less than -73ºC (-100ºF). Liquid nitrogen, liquid oxygen and carbon dioxide are the most common cryogenic materials used in the laboratory. Hazards may include fire, explosion, embrittlement, pressure buildup, frostbite and asphyxiation.
Many of the safety precautions observed for compressed gases also apply to cryogenic liquids. Two additional hazards are created from the unique properties of cryogenic liquids:
Extremely Low Temperatures –The cold boil-off vapor of cryogenic liquids rapidly freezes human tissue. Most metals become stronger upon exposure to cold temperatures, but materials such as carbon steel, plastics and rubber become brittle or even fracture under stress at these temperatures. Proper material selection is important. Cold burns and frostbite caused by cryogenic liquids can result in extensive tissue damage.
Vaporization - All cryogenic liquids produce large volumes of gas when they vaporize. Liquid nitrogen will expand 696 times as it vaporizes. The expansion ratio of argon is 847:1, hydrogen is 851:1 and oxygen is 862:1. If these liquids vaporize in a sealed container, they can produce enormous pressures that could rupture the vessel. For this reason, pressurized cryogenic containers are usually protected with multiple pressure relief devices.
 Vaporization of cryogenic liquids (except oxygen) in an enclosed area can cause asphyxiation. Vaporization of liquid oxygen can produce an oxygen-rich atmosphere, which will support and accelerate the combustion of other materials. Vaporization of liquid hydrogen can form an extremely flammable mixture with air.
Vaporization of cryogenic liquids (except oxygen) in an enclosed area can cause asphyxiation. Vaporization of liquid oxygen can produce an oxygen-rich atmosphere, which will support and accelerate the combustion of other materials. Vaporization of liquid hydrogen can form an extremely flammable mixture with air.
Cryogenic liquids have boiling points less than -73ºC (-100ºF). Liquid nitrogen, liquid oxygen and carbon dioxide are the most common cryogenic materials used in the laboratory. Hazards may include fire, explosion, embrittlement, pressure buildup, frostbite and asphyxiation.
Many of the safety precautions observed for compressed gases also apply to cryogenic liquids. Two additional hazards are created from the unique properties of cryogenic liquids:
General
Cryogenic liquids have boiling points less than -73ºC (-100ºF). Liquid nitrogen, liquid oxygen and carbon dioxide are the most common cryogenic materials used in the laboratory. Hazards may include fire, explosion, embrittlement, pressure buildup, frostbite and asphyxiation.
Many of the safety precautions observed for compressed gases also apply to cryogenic liquids. Two additional hazards are created from the unique properties of cryogenic liquids:
Extremely Low Temperatures –The cold boil-off vapor of cryogenic liquids rapidly freezes human tissue. Most metals become stronger upon exposure to cold temperatures, but materials such as carbon steel, plastics and rubber become brittle or even fracture under stress at these temperatures. Proper material selection is important. Cold burns and frostbite caused by cryogenic liquids can result in extensive tissue damage.
Vaporization - All cryogenic liquids produce large volumes of gas when they vaporize. Liquid nitrogen will expand 696 times as it vaporizes. The expansion ratio of argon is 847:1, hydrogen is 851:1 and oxygen is 862:1. If these liquids vaporize in a sealed container, they can produce enormous pressures that could rupture the vessel. For this reason, pressurized cryogenic containers are usually protected with multiple pressure relief devices.
Cryogenic liquids have boiling points less than -73ºC (-100ºF). Liquid nitrogen, liquid oxygen and carbon dioxide are the most common cryogenic materials used in the laboratory. Hazards may include fire, explosion, embrittlement, pressure buildup, frostbite and asphyxiation.
Many of the safety precautions observed for compressed gases also apply to cryogenic liquids. Two additional hazards are created from the unique properties of cryogenic liquids:
Hazards
Extreme ColdThe vapor of liquid nitrogen can rapidly freeze skin tissue and eye fluid, resulting in cold burns, frostbite, and permanent eye damage even by brief exposure.
Asphyxiation
Liquid nitrogen expands 696 times in volume when it vaporizes and has no warning properties such as odor or color. Hence, if sufficient liquid nitrogen is vaporized so as to reduce the oxygen percentage to below 19.5%, there is a risk of oxygen deficiency which may cause unconsciousness. Death may result if oxygen deficiency is extreme. To prevent asphyxiation hazards, handlers have to make sure that the room is well ventilated when using cryogens indoors.Oxygen Enrichment
When transferring liquid nitrogen, oxygen in the air surrounding a cryogen containment system can dissolve and create an oxygen-enriched environment as the system returns to ambient temperatures. Since the boiling point of nitrogen is lower than oxygen’s, liquid oxygen evaporates slower than nitrogen and may build up to levels which can increase the flammability of materials such as clothing near the system. Equipment containing cryogenic fluids must be kept clear of combustible materials in order to minimize the fire hazard potential. Condensed oxygen in a cold trap may combine with organic material in the trap to create an explosive mixture.
When transferring liquid nitrogen, oxygen in the air surrounding a cryogen containment system can dissolve and create an oxygen-enriched environment as the system returns to ambient temperatures. Since the boiling point of nitrogen is lower than oxygen’s, liquid oxygen evaporates slower than nitrogen and may build up to levels which can increase the flammability of materials such as clothing near the system. Equipment containing cryogenic fluids must be kept clear of combustible materials in order to minimize the fire hazard potential. Condensed oxygen in a cold trap may combine with organic material in the trap to create an explosive mixture.
Pressure Buildup and Explosions
Without adequate venting or pressure-relief devices on the containers, enormous pressures can build upon cryogen evaporation. Users must make sure that cryogenic liquids are never contained in a closed system. Use a pressure relief vessel or a venting lid to protect against pressure build-up.Handling
Prudent Safety Practices- Liquid nitrogen should be handled in well-ventilated areas.
- Handle the liquid slowly to minimize boiling and splashing. Use tongs to withdraw objects immersed in a cryogenic liquid - Boiling and splashing always occur when charging or filling a warm container with cryogenic liquid or when inserting objects into these liquids;
- Do not transport liquid nitrogen in wide-mouthed glass Dewars or Dewars not protected with safety tape;
- Use only approved containers. Impact resistant containers that can withstand the extremely low temperatures should be used. Materials such as carbon steel, plastic and rubber become brittle at these temperatures;
- Only store liquid nitrogen in containers with loose fitting lids (Never seal liquid nitrogen in a container). A tightly sealed container will build up pressure as the liquid boils and may explode after a short time;
- Never touch non-insulated vessels containing cryogenic liquids. Flesh will stick to extremely cold materials. Even nonmetallic materials are dangerous to touch at low temperatures;
- Never tamper or modify safety devices such as cylinder valve or regulator of the tank;
- Liquid nitrogen should only be stored in well-ventilated areas (do not store in a confined space);
- Do not store liquid nitrogen for long periods in an uncovered container;
- Cylinders and Dewars should not be filled to more than 80% of capacity, since expansion of gases during warming may cause excessive pressure buildup;
Personal Protective Equipment
Eye/face protectionA full face shield over safety glasses or chemical splash goggles are recommended during transfer and handling of cryogenic liquids to minimize injuries associated with splash or explosion.
Skin protection
Loose-fitting thermal insulated or leather gloves, long sleeve shirts, and trousers without cuffs should be worn while handling liquid nitrogen. Safety shoes are also recommended while handling containers.A special note on insulated gloves: Gloves should be loose-fitting, so they are able to be quickly removed if cryogenic liquid is spilled on them. Insulated gloves are not made to permit the hands to be put into a cryogenic liquid. They will only provide short-term protection from accidental contact with the liquid.
Liquid nitrogen/acetone (-94 °C) bath video manual
Traditional cooling baths
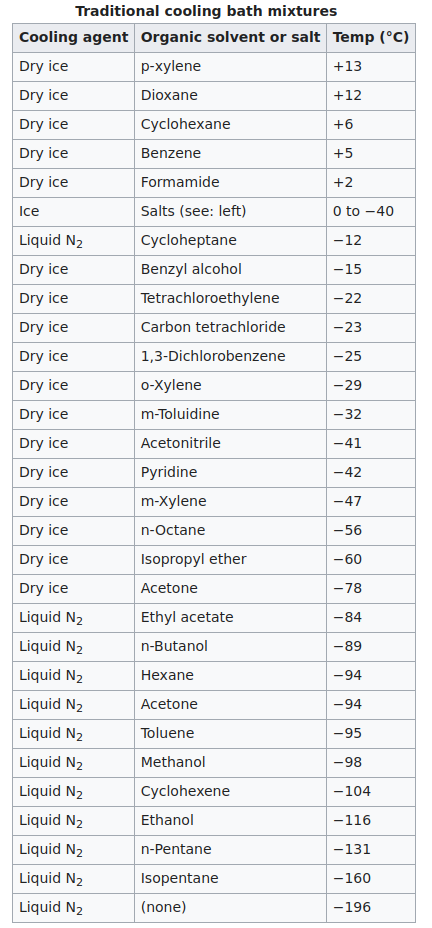
Water and ice baths
A bath of ice and water will maintain a temperature 0 °C, since the melting point of water is 0 °C. However, adding a salt such as sodium chloride will lower the temperature through the property of freezing-point depression. Although the exact temperature can be hard to control, the weight ratio of salt to ice influences the temperature:
A bath of ice and water will maintain a temperature 0 °C, since the melting point of water is 0 °C. However, adding a salt such as sodium chloride will lower the temperature through the property of freezing-point depression. Although the exact temperature can be hard to control, the weight ratio of salt to ice influences the temperature:
- −10 °C can be achieved with a 1:2.5 mass ratio of calcium chloride hexahydrate to ice.
- −20 °C can be achieved with a 1:3 mass ratio of sodium chloride to ice.
Dry ice baths at −78 °C
Since dry ice will sublime at −78 °C, a mixture such as acetone/dry ice will maintain −78 °C. Also, the solution will not freeze because acetone requires a temperature of about −93 °C to begin freezing. Therefore, other liquids with a lower freezing point (pentane: −95 °C, isopropyl alcohol: −89 °C) can also be used to maintain the bath at −78 °C.
Dry ice baths above −77 °C
In order to maintain temperatures above −77 °C, a solvent with a freezing point above −77 °C must be used. When dry ice is added to acetonitrile, the bath will begin cooling. Once the temperature reaches −41 °C, the acetonitrile will freeze. Therefore, dry ice must be added slowly to avoid freezing the entire mixture. In these cases, a bath temperature of −55 °C can be achieved by choosing a solvent with a similar freezing point (n-octane freezes at −56 °C).
Liquid-nitrogen baths above −196 °C
Liquid-nitrogen baths follow the same idea as dry-ice baths. A temperature of −115 °C can be maintained by slowly adding liquid nitrogen to ethanol until it begins to freeze (at −116 °C).
Water/ice alternatives
In water and ice-based baths, tap water is commonly used due to ease of access and the higher costs of using ultrapure water. However, tap water and ice derived from tap water can be a contaminant to biological and chemical samples. This has created a host of insulated devices aimed at creating a similar cooling or freezing effect as ice baths without the use of water or ice.
Since dry ice will sublime at −78 °C, a mixture such as acetone/dry ice will maintain −78 °C. Also, the solution will not freeze because acetone requires a temperature of about −93 °C to begin freezing. Therefore, other liquids with a lower freezing point (pentane: −95 °C, isopropyl alcohol: −89 °C) can also be used to maintain the bath at −78 °C.
Dry ice baths above −77 °C
In order to maintain temperatures above −77 °C, a solvent with a freezing point above −77 °C must be used. When dry ice is added to acetonitrile, the bath will begin cooling. Once the temperature reaches −41 °C, the acetonitrile will freeze. Therefore, dry ice must be added slowly to avoid freezing the entire mixture. In these cases, a bath temperature of −55 °C can be achieved by choosing a solvent with a similar freezing point (n-octane freezes at −56 °C).
Liquid-nitrogen baths above −196 °C
Liquid-nitrogen baths follow the same idea as dry-ice baths. A temperature of −115 °C can be maintained by slowly adding liquid nitrogen to ethanol until it begins to freeze (at −116 °C).
Water/ice alternatives
In water and ice-based baths, tap water is commonly used due to ease of access and the higher costs of using ultrapure water. However, tap water and ice derived from tap water can be a contaminant to biological and chemical samples. This has created a host of insulated devices aimed at creating a similar cooling or freezing effect as ice baths without the use of water or ice.
Last edited:
